

.jpg)

2024
Shanghai,June.3nd, 2024, Haihe Biopharma Co., Ltd. (hereby referred to as “Haihe Biopharma” or the “Company”) today announced that the clinical data of the paclitaxel oral solution and the PI3Kα inhibitor Risovalisib Mesylate were both presented in the poster section of 2024 American Society of Clinical Oncology (ASCO) Annual Meeting.
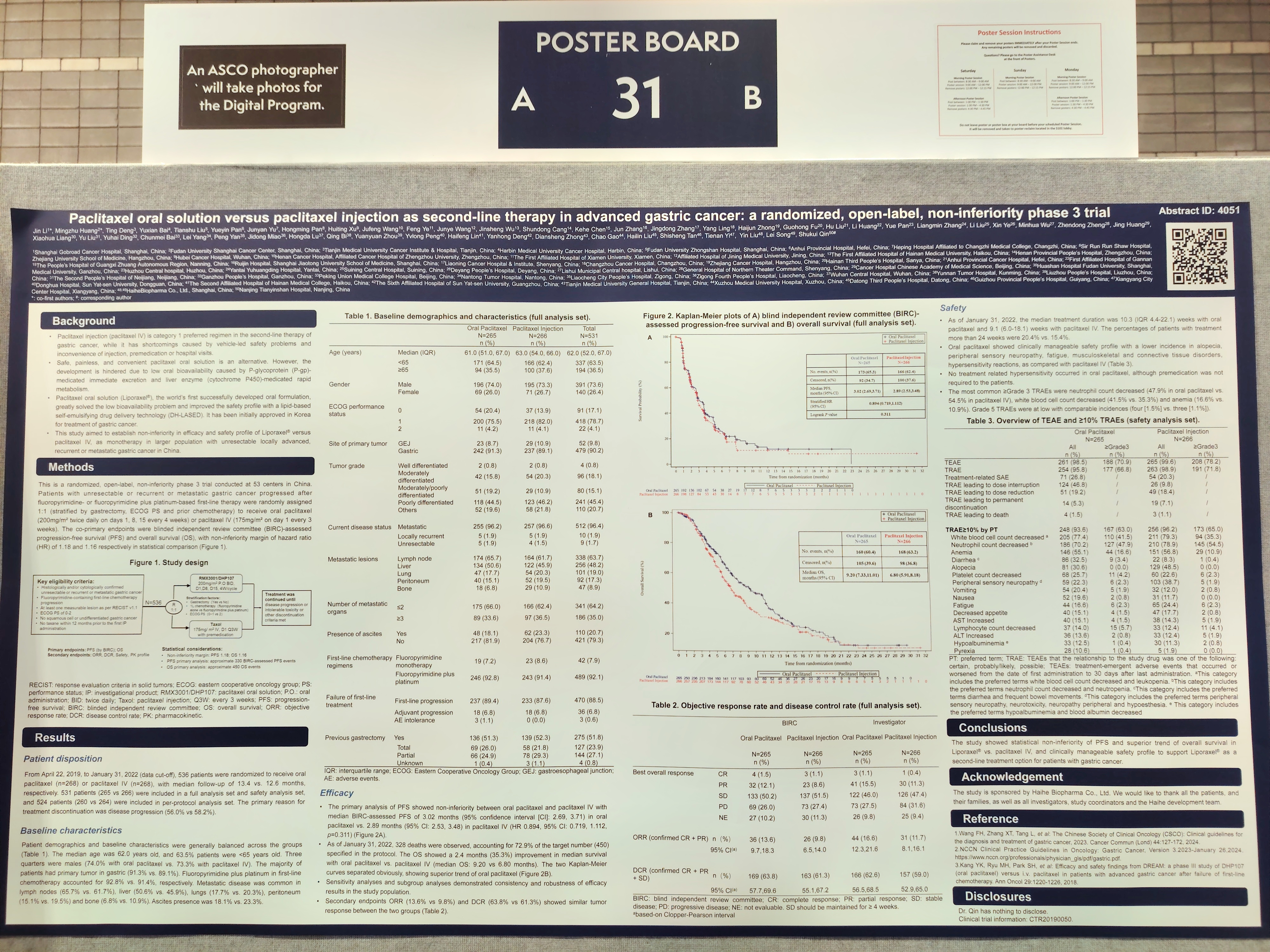
Title 1: Paclitaxel oral solution versus paclitaxel injection as second-line therapy in advanced gastric cancer: a randomized, open-label, non-inferiority phase 3 trial
Paclitaxel injection (paclitaxel IV) is category 1 preferred regimen in the second-line therapy of gastric cancer, while it has shortcomings caused by vehicle-led safety problems and inconvenience of injection, premedication or hospital visits. Paclitaxel oral solution, the world’s first successfully developed oral formulation, has been initially approved for treatment of gastric cancer.
This is a randomized, open-label, non-inferiority phase 3 trial aimed to determine non-inferiority in efficacy and safety profile of paclitaxel oral solution versus paclitaxel IV, as monotherapy in larger Chinese population with unresectable locally advanced, recurrent or metastatic gastric cancer after fluoropyrimidine- or fluoropyrimidine plus platinum-based first-line therapy failure. The co-primary endpoints were blind independent review committee (BIRC)-assessed progression-free survival (PFS) and overall survival (OS).
As of January 31, 2022 (data cut-off), 536 patients were randomized to receive paclitaxel oral solution (n=268) or paclitaxel IV (n=268), with median follow-up of 13.4 vs. 12.6 months, respectively. Patient demographics and baseline characteristics were balanced across the groups. The median age was 62.0 years old. Three quarters were males (74.0% with paclitaxel oral solution vs. 73.3% with paclitaxel IV). The majority of patients had primary tumor in gastric (91.3% vs. 89.1%). Fluoropyrimidine plus platinum in first-line chemotherapy accounted for 92.8% vs. 91.4%. Ascites presence was 18.1% vs. 23.3%.
The primary analysis of PFS showed non-inferiority between paclitaxel oral solution and paclitaxel IV with median BIRC-assessed PFS of 3.02 months in paclitaxel oral solution group vs. 2.89 months in paclitaxel IV group (HR 0.894, 95% CI: 0.719, 1.112, p=0.311). As of January 31, 2022, 328 deaths were observed, accounting for 72.9% of the target number (450). The OS showed a 2.4 months (35.3%) improvement in median survival for paclitaxel oral solution (median OS: 9.20 vs 6.80 months) and the OS Kaplan-Meier curves separated obviously, showing superior trend of paclitaxel oral solution. Secondary endpoints ORR (13.6% vs 9.8%) and DCR (63.8% vs 61.3%) showed similar tumor response between the two groups.
For the treatment-related adverse events (TRAEs), paclitaxel oral solution decreased neuropathy peripheral incidence (dose-limiting toxicity of paclitaxel product; 22.3% vs. 38.7% all grade) and no hypersensitivity occurred without premedication. The most common ≥Grade 3 TRAEs were neutrophil count decreased (47.9% in paclitaxel oral solution vs. 54.5% in paclitaxel IV), white blood cell count decreased (41.5% vs. 35.3%) and anemia (16.6% vs. 10.9%). Grade 5 TRAEs were at low with comparable incidences (four [1.5%] vs. three [1.1%]).
The study showed statistical non-inferiority of PFS and superior trend of overall survival in paclitaxel oral solution vs. paclitaxel IV, and clinically manageable and improved safety profile with lower incidence in alopecia, neuropathy peripheral, fatigue, musculoskeletal and connective tissue disorders, hypersensitivity reactions compared with paclitaxel IV, to support paclitaxel oral solution as a second-line treatment option for patients with gastric cancer.
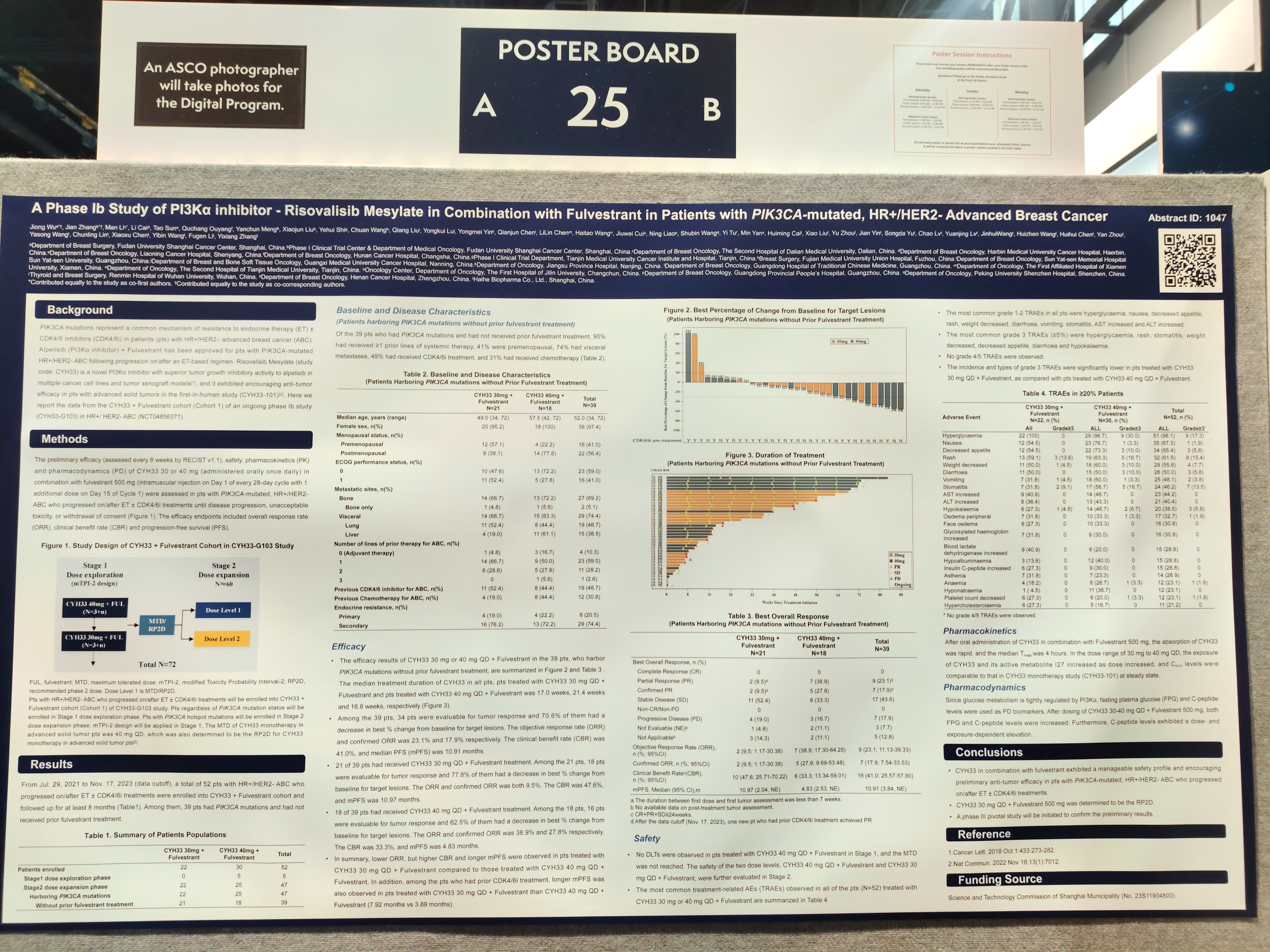
Title 2: A Phase Ib Study of PI3Kα inhibitor - Risovalisib Mesylate in Combination with Fulvestrant in Patients with PIK3CA-mutated, HR+/HER2- Advanced Breast Cancer
PIK3CA mutations represent a common mechanism of resistance to endocrine therapy (ET) ± CDK4/6 inhibitors (CDK4/6i) in patients (pts) with HR+/HER2– advanced breast cancer (ABC). Risovalisib Mesylate (study code: CYH33) is a novel PI3Kα inhibitor with superior tumor growth inhibitory activity in multiple cancer cell lines and tumor xenograft models, and it exhibited encouraging anti-tumor efficacy in pts with advanced solid tumors in the first-in-human study (CYH33-101). Here we report the data from the CYH33 + Fulvestrant cohort of an ongoing phase Ib study (CYH33-G103) in HR+/ HER2- ABC.
A total of 52 pts with HR+/HER2- ABC who progressed on/after ET ± CDK4/6i treatments were enrolled into CYH33 + Fulvestrant cohort. Among them, 39 pts had PIK3CA mutations and had not received prior fulvestrant treatment. Among the 39 pts, 90% had received ≥1 prior lines of systemic therapy, 41% were premenopausal, 74% had visceral metastases, 49% had received CDK4/6i treatment, and 31% had received chemotherapy.
Efficacy Results: Among the 39 pts, the median PFS (mPFS) was 10.91 months. 21 of 39 pts had received CYH33 30 mg QD + Fulvestrant (RP2D) treatment. Among the 21 pts, the mPFS was 10.97 months. For pts with prior CDK4/6i treatment at RP2D, the mPFS was 7.92 months.
Safety Results: The most common grade 1-2 TRAEs in all pts (N=52) were hyperglycaemia, nausea, decreased appetite, rash, weight decreased, diarrhoea, vomiting, stomatitis, AST increased, and ALT increased. The most common grade 3 TRAEs were hyperglycaemia, rash and stomatitis. No grade 4/5 TRAEs were observed.
CYH33 in combination with fulvestrant exhibited a manageable safety profile and encouraging preliminary anti-tumor efficacy in pts with PIK3CA-mutated, HR+/HER2- ABC who progressed on/after ET ± CDK4/6i treatments.
The American Society of Clinical Oncology (ASCO) is the world's leading academic organization for the specialty of oncology. The organization has nearly 40,000 members in more than 100 countries. ASCO annual meeting is the highest-level meeting in the field of clinical oncology. Many important research findings and clinical trial results are presented at ASCO's annual meeting.
Paclitaxel oral solution (study code: RMX3001; Korean code: DHP107) is an oral paclitaxel formulation developed by DAEHWA Pharmaceutical based on its innovative technology of lipid self-emulsifying drug delivery (DH-LASED). It has been approved for the second-line treatment of advanced and metastatic or local recurrent gastric cancer by the South Korean Ministry of Food and Drug Safety (MFDS) on September 9, 2016, with the trade name of Liporaxel®. To date, Liporaxel® is the first oral paclitaxel product that has been successfully developed and approved in the world, and the clinical development in breast cancer is ongoing in US, Europe and Asia. In September 2017, Haihe Biopharma obtained the rights of the product in mainland China, Taiwan, Hong Kong, and Thailand from DAEHWA Pharmaceutical.
Risovalisib Mesylate (study code: CYH33) is a novel, highly potent, selective inhibitor of alpha subunit of phosphatidylinositol 3-kinase (PI3Kα) with intellectual property rights by Haihe Biopharma. Preclinical studies have demonstrated that CYH33 potently inhibited the activity of PI3Kα, and it exhibits remarkable anti-tumor activity both in vitro and in vivo. Clinical studies have shown that CYH33 monotherapy or in combination with other anti-tumor drugs have shown preliminary promising anti-tumor efficacy in patients with advanced solid tumors, especially those carrying PIK3CA hotspot mutations.
Haihe Biopharma Co., Ltd is a global, values-based, R&D driven biopharmaceutical leader headquartered in China with operation centers in the US and Japan, and mainly focuses on innovative anti-tumor therapies. The company has the comprehensive capability of drug discovery, development, manufacturing and commercialization, and delivers life-saving therapies to cancer patients in China and around the world. As a R&D focused company with many experts skilled in developing new drugs, Haihe Biopharma is committed in-house innovation with global perspective management and R&D team. The Company currently has one approved product (INN: Gumarontinib) in China and several drug candidates under development.